Billionaire Elon Musk announced that his company Neuralink has implanted a chip into the human brain for the first time after passing the extremely rigorous licensing process of US regulators.
According to billionaire Elon Musk , Neuralink ‘s first chip implant into the human brain was recently performed and the implanted person is recovering well. If researched successfully, the company believes this technology will allow people with chip implants to control devices such as phones solely through their minds. Technology can also be used to treat neurological diseases.

Billionaire Elon Musk and the surgical robot used to implant Neuralink chips
AFP
How does the device work?
In May 2023, Neuralink received approval from the US Food and Drug Administration to conduct chip delivery testing in the human brain. The purpose of the trial was to evaluate the safety of the device and of the surgical robot that performed the implantation, as well as the device’s ability to read and decode neural activity.
Neuralink is a neural technology company founded by billionaire Elon Musk in 2016 with the goal of building link channels. According to Axios , the device, whose current design is about the size of a coin, is said to be “aesthetically invisible”, consisting of a wirelessly rechargeable lithium battery, tiny computer chips and other components. other electronic parts.
A surgical robot like a sewing machine, with cameras, sensors and needles smaller than a human hair, is used to implant the device into the human brain.

Neuralink’s brain-implantable device is about the size of a coin
AFP
Neuralink ‘s first product will be called Telepathy, helping people control phones, computers and most other devices with just their thoughts. The first potential customers will be people who have lost their hands or have diseases that prevent them from moving their hands. “Imagine Stephen Hawking being able to communicate faster than a typist or an auctioneer. That’s the goal,” Mr. Elon Musk said, referring to the famous physical scientist who suffers from degenerative muscle disease.
Elon Musk’s Neuralink has implanted a chip in the human brain
In 2021, Neuralink once let a chip-implanted monkey demonstrate the ability to control a computer mouse cursor and play a video game using brain signals. However, the company has been accused of animal abuse and of risking humans contracting infectious diseases through contaminated hardware.
According to Reuters, company employees made internal comments that animal testing was rushed, causing animals to suffer and die, leading to an investigation by the US Department of Agriculture. According to records, the company has killed 1,500 animals since 2018, including 280 sheep, pigs and monkeys. Neuralink defended the company’s treatment of animals while Mr. Musk once stated that no monkeys have died due to chip implantation.
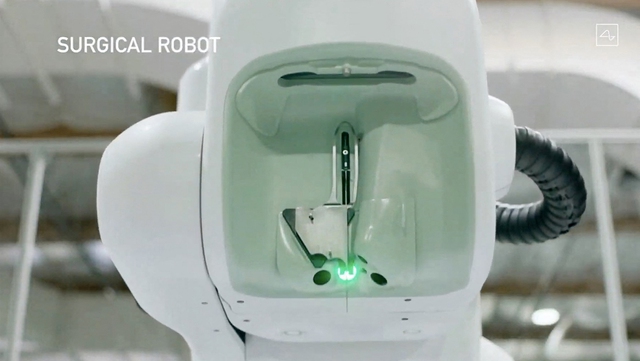
Neuralink’s surgical robot AFP
How does Neuralink apply for a license?
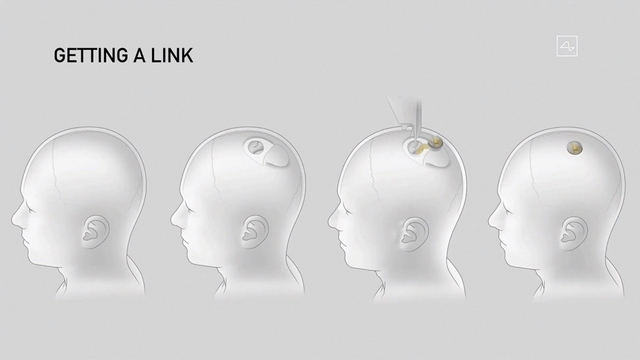
Simulate Neuralink’s steps to implant a device into the human brain
AFP
In May 2023, Neuralink announced that it had been approved by the US Food and Drug Administration ( FDA ) for testing chip implantation in the human brain and the FDA also confirmed this information. Previously, the company was denied a license for an undisclosed reason.
In order for new medical devices like Neuralink’s chip to hit the market, the company must apply for an investigational device exemption (IDE) with the FDA. This application must typically include detailed information about the device, the purpose of the trial, the clinical trial plan, and assurances for safety and effectiveness.
American newspaper: Billionaire Musk regularly uses banned substances
According to Axios , Neuralink may have applied for licenses for implantable devices, surgical robots and related software. Neuralink’s devices are considered to pose a high risk and will require additional management steps.
If the FDA determines that the trial was not performed as described in the application or caused serious harm to participants, the agency may stop or completely terminate the clinical trial at any time. any.

Neuralink’s device is expected to help people control electronic devices with their thoughts and can help cure neurological diseases.
REUTERS
In order for this device to be licensed for market release, the company must go through the premarket approval (PMA) process.
During this process, FDA scientists will thoroughly examine trial results and other elements of the license application before approval. The agency may also exclude certain patient groups or limit the use of the device to patients with specific medical needs.
News
LORD OF SKY: STEPHEN CURRY SPLASHES OUT $4 MILLION TO BUY A PRIVATE JET FOR TRAVELING WITH HIS WIFE AND DAUGHTER
NBA player Stephen Curry is a fantastic family man in addition to being a fantastic basketball player. He routinely takes time off from his hectic work to travel the world on their private plane with his adored daughter Riley. The…
BIG LOVE: BASKETBALL SUPERSTAR DRAYMOND GREEN SPENT HUGE SUMS ON A PRIVATE JET AS A BIRTHDAY GIFT FOR HIS WIFE FOR HER 38TH BIRTHDAY, INSIDE DETAILS REVEALED
Basketball star Draymond Green buys a gorgeous $12 million private plane as a surprise for his wife’s 30th birthday. It’s an incredibly costly gift. This amazing present demonstrates Draymond’s unwavering love and gratitude for his spouse as well as his…
EVERY EXPERT AGREES THAT STEPHEN CURRY IS THE GREATEST GOLDEN STATE WARRIOR OF ALL TIME
Stephen Curry, the electrifying point guard of the Golden State Warriors, stands as the embodiment of excellence and innovation in basketball. With a combination of unmatched shooting prowess, unparalleled ball-handling skills, and remarkable leadership, Curry has redefined the game. His…
SOCIAL MEDIA IS DROOLING OVER A PHOTO OF WNBA STAR CAITLIN CLARK WEARING AN ASTONISHINGLY SHORT DRESS, SHOWING OFF LONGER LEGS THAN SHE’S EVER SHOWN BEFORE (PIC)
Caitlin Clark (Photo by Sarah Stier/Getty Images) Caitlin Clark, the all-time NCAA Division 1 basketball scoring leader, is the face of the NCAA and a perennial cash cow to any and everything she comes into contact with. The regional final matchup between…
CLIPPERS’ PATRICK PATTERSON UNDER FIRE FOR CALLING BLACK WOMEN ‘BULLDOGS’ WHEN ASKED WHY NBA PLAYERS MARRY OTHER RACES (PIC)
Patrick Patterson is on the hot seat aftr his comments sent social media into a frenzy. The Los Angeles Clippers forward married his girlfriend earlier this year, but when one fan saw his wife, he questioned him on it. This…
SHAQUILLE O’NEAL SPOTTED OUT IN LOS ANGELES WITH FAMOUS BASKETBALL GROUPIE BRITTANY RENNER (PIC + VIDEO)
LAS VEGAS, NEVADA – JANUARY 07: Shaquille O’Neal demos Amazon Alexa in the Lamborghini Huracan Evo during the Amazon After Hours during CES 2020 at The Venetian Las Vegas on January 07, 2020 in Las Vegas, Nevada. (Photo by Roger…
End of content
No more pages to load











